Authenticate Your Data...
Ensure your data is compliant with 21 CFR Part 11 requirements with the XPRT Part 11 software.
21 CFR Part 11 requires that all data submitted to the FDA be
authenticated to ensure that it has not been altered in any way.
Under Part 11, electronic records and electronic signatures may be
equivalent to paper records and traditional handwritten signatures.
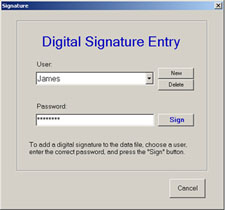
XPRT Part 11 software helps users meet these requirements by applying an
automatic, unalterable digital signature to all created data files. If the data file
is corrupted in any way, the signature will be removed and the user will not
be able to sign the data file again. This feature identifies files that have been corrupted or altered.
For more information about XPRT Part 11 software and its compliance with CFR
Part 11, please contact our office.